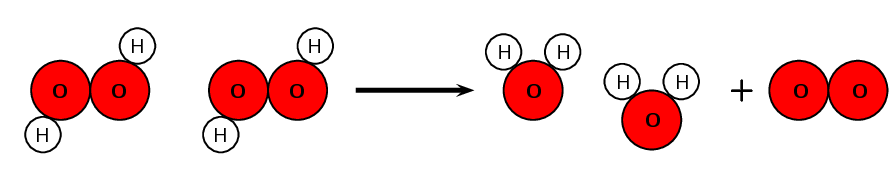
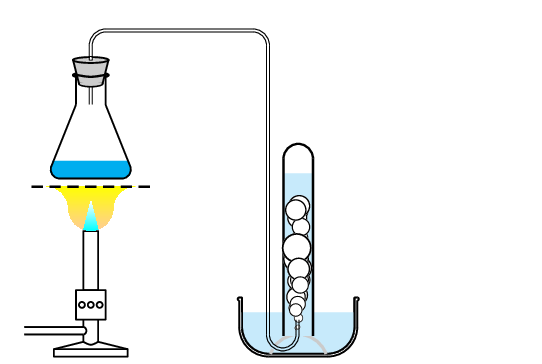
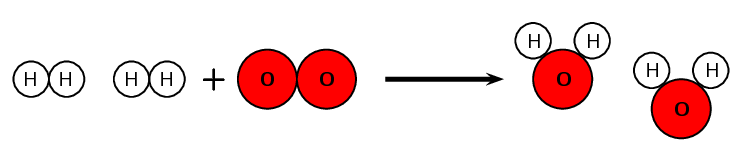
For each of the following say whether a chemical or a physical change occurs.
- Melting candle wax.
- Mixing sodium chloride (\(\text{NaCl}\)) and silver nitrate (\(\text{AgNO}_{3}\)) to form silver chloride (\(\text{AgCl}\)).
- Mixing hydrochloric acid (\(\text{HCl}\)) and magnesium ribbon (\(\text{Mg}\)) to form magnesium chloride (\(\text{MgCl}_{2}\)).
- Dissolving salt in water.
- Tearing a piece of magnesium ribbon.
- Physical change
- Chemical change
- Chemical change
- Physical change
- Physical change